SABR/SBRT In Oligometastatic Cancer. Dr Aznar
The term oligometastasis refers to patients with a limited number of metastatic sites (usually considered patients with ≤5 metastatic sites). Historically, once a cancer has acquired the capacity to metastasize, it has generally been considered incurable, with a few rare chemotherapy-sensitive cases. Since 1995, an increasing number of cases have been identified in which cancer has metastasized (≤5), but management includes local ablative therapy because survival rates are closer to pre-metastatic stages.
Inclusion criteria for SABR/SBRT in oligometastases (SABR Consortium UK): All of the following criteria must be met: primary histology, 1-3 metastatic sites technically amenable to SABR (NCCN: 1-5), lesions ≤5 cm each, patient not amenable to surgery/inappropriate, disease-free interval (DFI) >6 months from the primary, life expectancy >6 months, PS ≤2, a maximum of two sites of spinal metastatic disease, all patients to be discussed for stereotactic MDT with the presence of, or after discussion with a disease-site-specific oncologist, a recent body CT or PET scan.
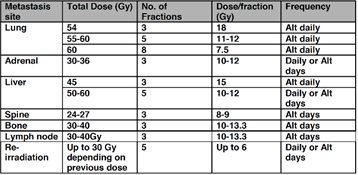
Dose and fractionation by location of oligometastases: The most common locations for SABR therapy for oligometastases are lung, liver, vertebrae, lymph nodes, and bone. The most frequently used doses and fractionation are described in the table (NHS England SABR Commissioning Policy).
Volumes and margins: In bone, the lesion is delineated as a GTV on the planning CT; if necessary, MRI can be used as an aid. A 3 mm margin can be added to create the CTV. A 5 mm margin is usually added to create the PTV (this is a general rule for SABR with linac; CK may use smaller margins). For adrenal gland metastases, motion control maneuvers, IGRT, and margins are the same as those used in other upper abdominal locations such as the liver or pancreas (described below). When treating lymph nodes, the node is delineated as a CTV on the planning CT (preferably with contrast to differentiate vessels from nodes), and a 5 mm margin is added. Immobilization and use of oral contrast agents vary depending on the location of the node.
In SABR re-irradiation, an organ-by-organ radiobiological calculation must be performed to determine the decay and dose that each healthy organ can tolerate in the current SABR treatment.
BIBLIOGRAPHIC REFERENCES.
- Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy. A Comprehensive Guide. Trifiletti D, Chao SE, Sahgal A, Sheehan JP. Springer. 2019
- LEKSELL L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand 1951; 102:316
- SABR Consortium UK, 2016 and SABR Consortium UK, 2019
- NCCN guidelines available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- Potters L, Kavanagh B, Galvin JM, et al. American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2010; 76:326.
